Simulation of oncogenic proteins by Molecular Dynamics and in silico drug design
Jan 14, 2025
Zheyao Hu, a student with a scholarship from the China Scholarship Council, defended her doctoral thesis entitled “In silico design of inhibitors of RAS oncogenic proteins and strategies for blocking of tumour growth” and directed by Dr. Jordi Martí Rabassa on January 14, 2025 at the UPC North Campus. The thesis presents a detailed study of the behavior of oncogenes of the RAS family, related to a third of all cancers that exist, and proposes a new methodology to achieve the computer design of drugs capable of blocking the activation of oncogenes. As an example, two prototype drugs are proposed, one of them potentially suitable for treating patients with pancreatic cancer and another for patients with melanoma
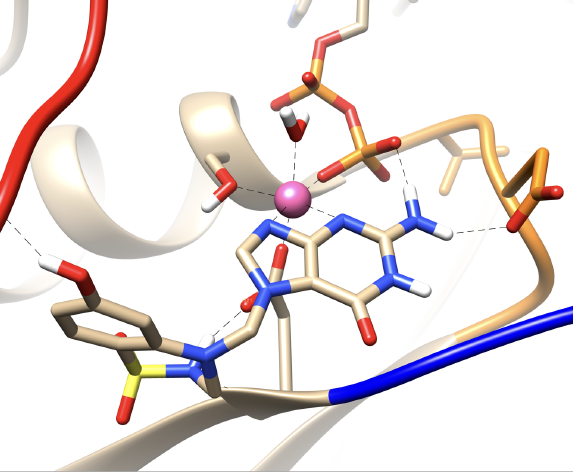
This Doctoral Thesis is dedicated to the study of the structural properties of RAS oncogenes, which have a fundamental influence on 30% of human tumours, mainly pancreatic, lung, colon and skin cancers. RAS proteins are a family of important molecular regulators that play a key role in a wide variety of cellular functions such as proliferation, differentiation, adhesion or apoptosis. These proteins are binary switches between guanosine-diphosphatase (GDP) and guanosine-triphosphatase, being able to regulate cytoplasmic signalling networks, essential in the growth, differentiation and survival of cells.
From the technical point of view, classical molecular dynamics simulations (on a scale of 10 microseconds) and well-tempered metadynamics (WTM) simulations have been applied, so that all the systems considered have been modelled at a fully atomic level, with systems up to 250000 atoms. The application of WTM simulations of 1 microsecond, has allowed us to perform for the first-time free energy calculations related to the union of various molecular probes with models of cell membranes in aqueous solution. The investigated free energy surfaces show a specific behaviour of the bonds of small molecules, such as benzothiadiazine, in phospholipid membranes.
Although KRAS-G12D mutations are one of the most frequent oncogenic drivers in human cancers, no therapeutic agent directly targeting KRAS-G12D has yet been clinically approved, which remains drug-free. We have discovered that the cofactor Mg2+ plays a crucial role in the conformational changes of the KRAS-GDP complex, we have located two novel pharmacological dynamic pockets unique to KRAS-G12D, and we have designed in silico the inhibitor DBD15-21-22, which can target -se specifically and closely to the KRAS-G12D-GDP-Mg2+ ternary complex providing a suitable strategy for its inhibition. Another inhibitor (HM-387) has also been proposed to treat tumours related to the NRAS-Q61R oncogene.
Share: